Details of the Drug
General Information of Drug (ID: DMMIQ7G)
| Drug Name |
Rosuvastatin
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Astende; Cirantan; Cresadex; Creston; Crestor; Provisacor; Razel; Rosedex; Rosimol; Rosumed; Rosustatin; Rosuvas; Rosuvast; Rosvel; Rovartal; Simestat; Sinlip; Vivacor; Rosuvastatin [INN]; Rosuvastatin calcium; Rosuvastatin calcium [USAN]; Rosuvastatin hemicalcium; S 4522; ZD 4522; ZD4522; AZD-4522; Creston (TN);Crestor (TN); Pyrimidine Compound, 26; Rosuvastatin (INN); S-4522; ZD 4522, calcium salt; ZD-4522; Rosuvastatin calcium (JAN/USAN); Bis[(E)-7-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino] pyrimidin-5-yl](3R,5S)-3,5-dihydroxyhelpt-6-enoic acid] calcium salt; Calcium (E,3R,5S)-7-[4-(4-fluorophenyl)-2-[methyl(methylsulfonyl)amino]-6-propan-2-ylpyrimidin-5-yl]-3,5-dihydroxyhept-6-enoate; (3R,5S,6E)-7-(4-(4-fluorophenyl)-6-(1-methylethyl)-2-(ethyl(methylsulfonyl)amino)-5-pyrimidinyl)-3,5-dihydroxy-6-heptenoic acid; (3R,5S,6E)-7-{4-(4-fluorophenyl)-2-[methyl(methylsulfonyl)amino]-6-(propan-2-yl)pyrimidin-5-yl}-3,5-dihydroxyhept-6-enoic acid; (3R,5S,6E)-7-{4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino]pyrimidin-5-yl}-3,5-dihydroxyhept-6-enoic acid; (E,3R,5R)-7-[4-(4-fluorophenyl)-2-[methyl(methylsulfonyl)amino]-6-propan-2-ylpyrimidin-5-yl]-3,5-dihydroxyhept-6-enoic acid; (E,3R,5S)-7-[4-(4-fluorophenyl)-2-[methyl(methylsulfonyl)amino]-6-propan-2-ylpyrimidin-5-yl]-3,5-dihydroxyhept-6-enoic acid; (S-((R*,S*-(E)))-7-(4-(4-fluorophenyl)-6-(1-methylethyl)-2-(methyl(methylsulfonyl) amino)-5-pyrimidinyl)-3,5-dihydroxy-6-heptenoic acid, calcium salt (2:1); (S-(R*,S*-(E)))-7-(4-(4-Fluorophenyl)-6-(1-methylethyl)-2-(methyl(methylsulfonyl)amino)-5-pyrimidinyl)-3,5-dihydroxy-6-heptenoic acid, calcium salt (2:1); 6-Heptenoic acid, 7-(4-(4-fluorophenyl)-6-(1-methylethyl)-2-(ethyl(methylsulfonyl)amino)-5-pyrimidinyl)-3,5-dihydroxy-, (3R,5S,6E); 6-Heptenoic acid, 7-(4-(4-fluorophenyl)-6-(1-methylethyl)-2-(methyl(methylsulfonyl)amino)-5-pyrimidinyl)-3,5-dihydroxy-, calcium salt (2:1), (3R,5S,6E)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Anticholesteremic Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
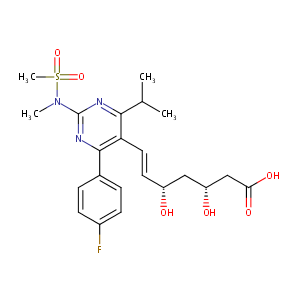 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 481.5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 10 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 10 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Arteriosclerosis | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | BD40 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Rosuvastatin
Coadministration of a Drug Treating the Disease Different from Rosuvastatin (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Rosuvastatin FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2954). | ||||
| 3 | Martin PD, Warwick MJ, Dane AL, Hill SJ, Giles PB, Phillips PJ, Lenz E: Metabolism, excretion, and pharmacokinetics of rosuvastatin in healthy adult male volunteers. Clin Ther. 2003 Nov;25(11):2822-35. | ||||
| 4 | BDDCS predictions, self-correcting aspects of BDDCS assignments, BDDCS assignment corrections, and classification for more than 175 additional drugs | ||||
| 5 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 6 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 7 | Association of the Trp719Arg polymorphism in kinesin-like protein 6 with myocardial infarction and coronary heart disease in 2 prospective trials: the CARE and WOSCOPS trials. J Am Coll Cardiol. 2008 Jan 29;51(4):435-43. doi: 10.1016/j.jacc.2007.05.057. | ||||
| 8 | ABCG2 polymorphism is associated with the low-density lipoprotein cholesterol response to rosuvastatin. Clin Pharmacol Ther. 2010 May;87(5):558-62. | ||||
| 9 | Pharmacogenetic study of statin therapy and cholesterol reduction. JAMA. 2004 Jun 16;291(23):2821-7. doi: 10.1001/jama.291.23.2821. | ||||
| 10 | New dimension of statin action on ApoB atherogenicity. Clin Cardiol. 2003 Jan;26(1 Suppl 1):I7-10. | ||||
| 11 | Drug and bile acid transporters in rosuvastatin hepatic uptake: function, expression, and pharmacogenetics. Gastroenterology. 2006 May;130(6):1793-806. | ||||
| 12 | Involvement of multiple transporters in the hepatobiliary transport of rosuvastatin. Drug Metab Dispos. 2008 Oct;36(10):2014-23. | ||||
| 13 | The contribution of organic anion transporters OAT1 and OAT3 to the renal uptake of rosuvastatin. J Pharmacol Exp Ther. 2007 Sep;322(3):1221-7. | ||||
| 14 | Effects of acid and lactone forms of eight HMG-CoA reductase inhibitors on CYP-mediated metabolism and MDR1-mediated transport. Pharm Res. 2006 Mar;23(3):506-12. | ||||
| 15 | Pharmacokinetics of rosuvastatin when coadministered with rifampicin in healthy males: a randomized, single-blind, placebo-controlled, crossover study. Clin Ther. 2008 Jul;30(7):1283-9. | ||||
| 16 | Product Information. Nexletol (bempedoic acid). Esperion Therapeutics, Ann Arbor, MI. | ||||
| 17 | Product Information. Crestor (rosuvastatin). AstraZeneca Pharma Inc, Mississauga, ON. | ||||
| 18 | Product Information. Kynamro (mipomersen). Genzyme Corporation, Cambridge, MA. | ||||
| 19 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 20 | Product Information. Juxtapid (lomitapide). Aegerion Pharmaceuticals Inc, Cambridge, MA. | ||||
| 21 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 22 | Product Information. Piqray (alpelisib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 23 | Product Information. Prevymis (letermovir). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 24 | Product Information. Olysio (simeprevir). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 25 | Product Information. Epclusa (sofosbuvir-velpatasvir). Gilead Sciences, Foster City, CA. | ||||
| 26 | Product Information. Accolate (zafirlukast). Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 27 | Product Information. Rukobia (fostemsavir). ViiV Healthcare, Research Triangle Park, NC. | ||||
| 28 | Neuvonen PJ, Niemi M, Backman JT "Drug interactions with lipid-lowering drugs: Mechanisms and clinical relevance." Clin Pharmacol Ther 80 (2006): 565-81. [PMID: 17178259] | ||||
| 29 | Product Information. Tabrecta (capmatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 30 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 31 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 32 | Product Information. Varubi (rolapitant). Tesaro Inc., Waltham, MA. | ||||
